Clinical Trials & Pipeline
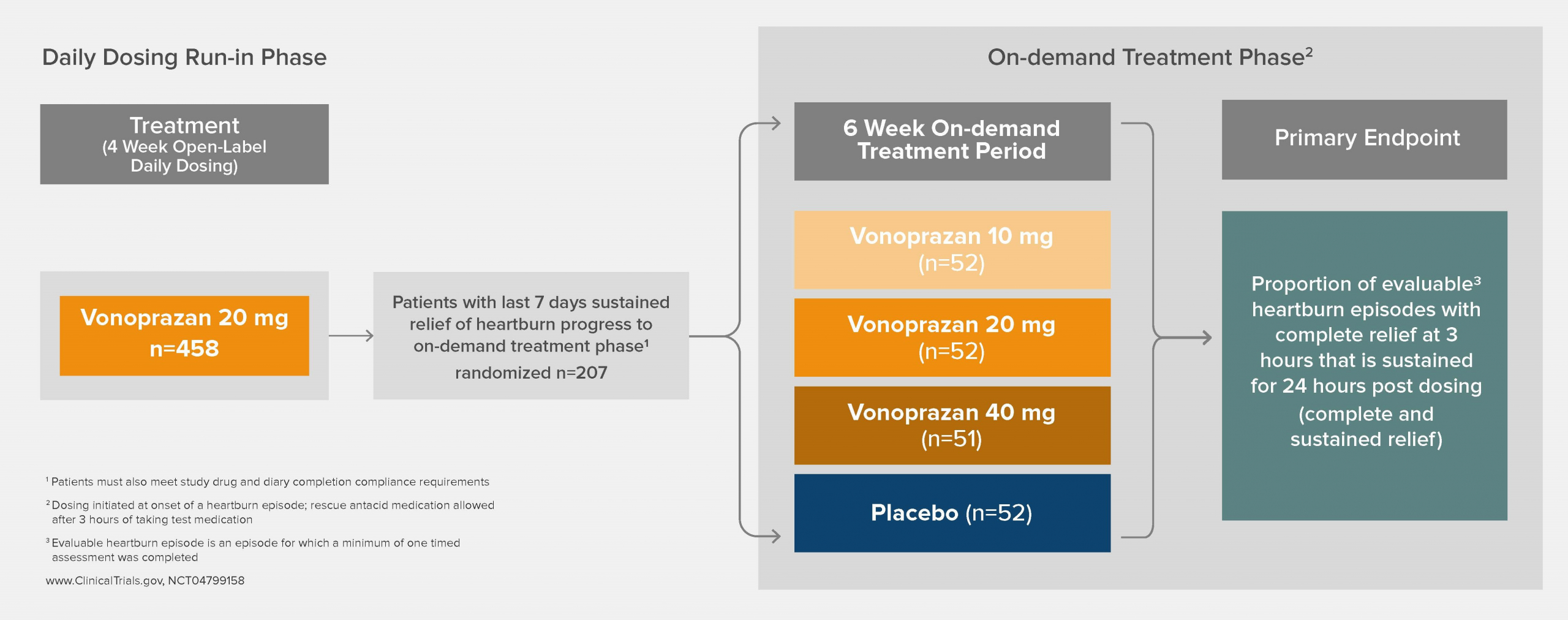
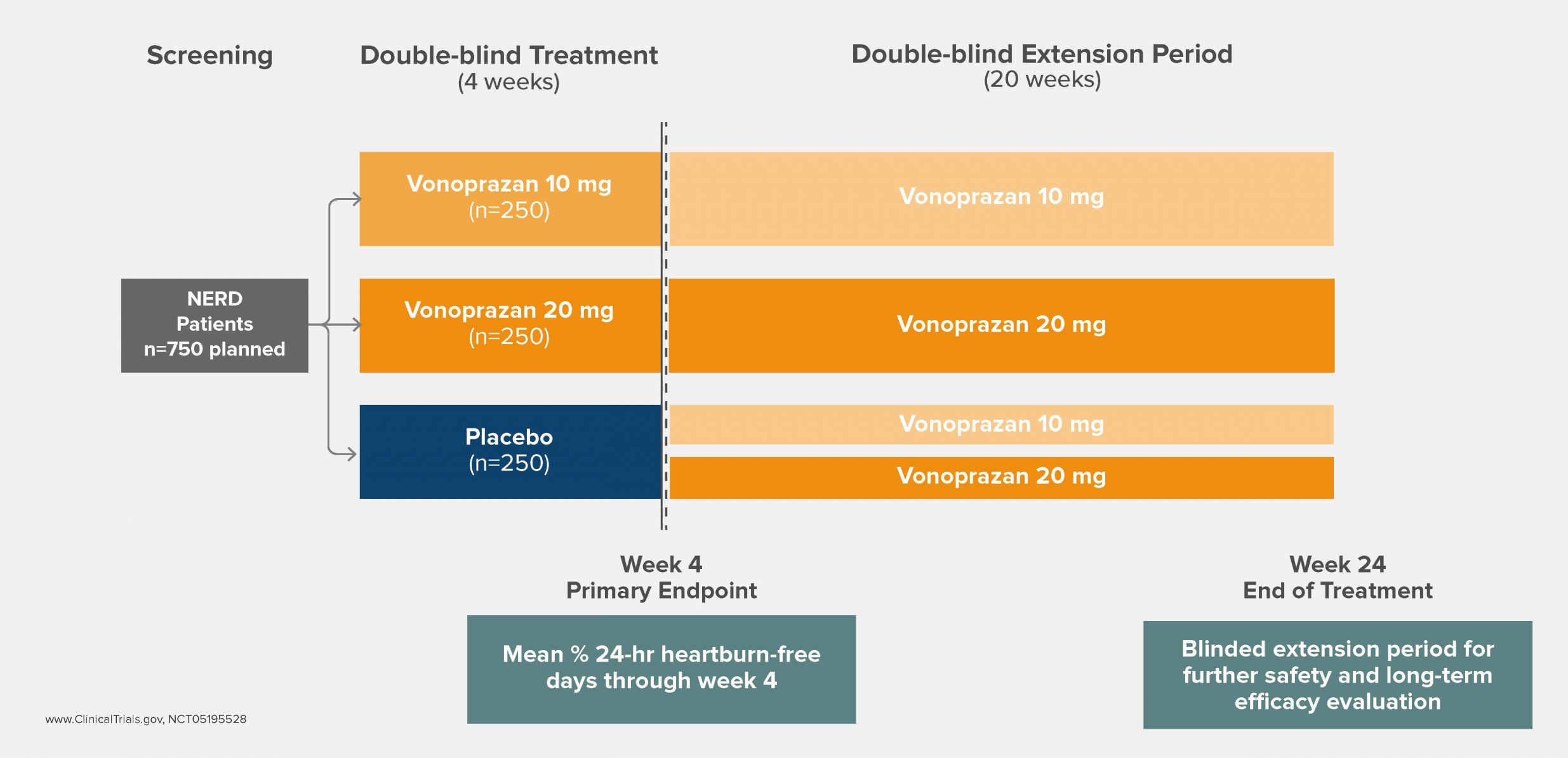
Phathom Pharmaceuticals is advancing its development program across multiple acid-related GI disorders, including Non-Erosive GERD (NERD) and Eosinophilic Esophagitis (EoE).
Phathom's Expanded Access Policy
At Phathom, we work every day to improve the lives of people impacted by gastrointestinal diseases and to make therapies available to patients as quickly and safely as possible. Expanded access, also referred to as compassionate use, is a channel through which the US Food and Drug Administration (FDA) allows patients to receive investigational medicines in certain circumstances outside of the context of a clinical trial.
We do not currently make any of our investigational products available through expanded access programs, as we believe that our clinical trials are the most appropriate way to access our investigational products. We encourage patients who are interested in accessing therapies in our pipeline to talk to their doctor about participating in a clinical trial. Information about all of our trials, including enrollment status, eligibility criteria, and locations, is available at ClinicalTrials.gov.
To the extent we determine that expanded access to any of our investigational therapies becomes appropriate in the future, this website and policy will be updated to include information regarding the criteria to be used to evaluate these requests, among other information. If you have further questions, please contact our Medical Information team. We anticipate that your inquiry will be acknowledged within five business days.
Phathom Pharmaceuticals is going beyond the expected and working to change the GI treatment landscape for millions of patients suffering from acid-related disorders.
June 2025 US-PHAT-25-0027